Summary Card
Definition
A malignant tumour of melanocytes.
Risk Factors for Melanoma
Environment (radiation), genetic (BRAF) and patient-related factors (skin type and medical history).
Diagnosis
Clinical suspicion is confirmed with a histological diagnosis.
Types of Melanoma
Superficial spreading, nodular, lentigo, acral, subungual, amelanocytic and desmoplastic.
Wide Local Excision of Melanoma
Margins are dependent on the Breslow thickness.
Sentinel Lymph Node Biopsy
Indicated ≥ pT2a or ≥ pT1b with high-risk features. Techniques include radioactive isotope, blue dye and indocyanine green.
Management of a +SLNB
Three options: complete lymph node dissection, observation, and adjuvant therapies.
Staging Melanoma
TNM classification from the AJCC 8th Edition.
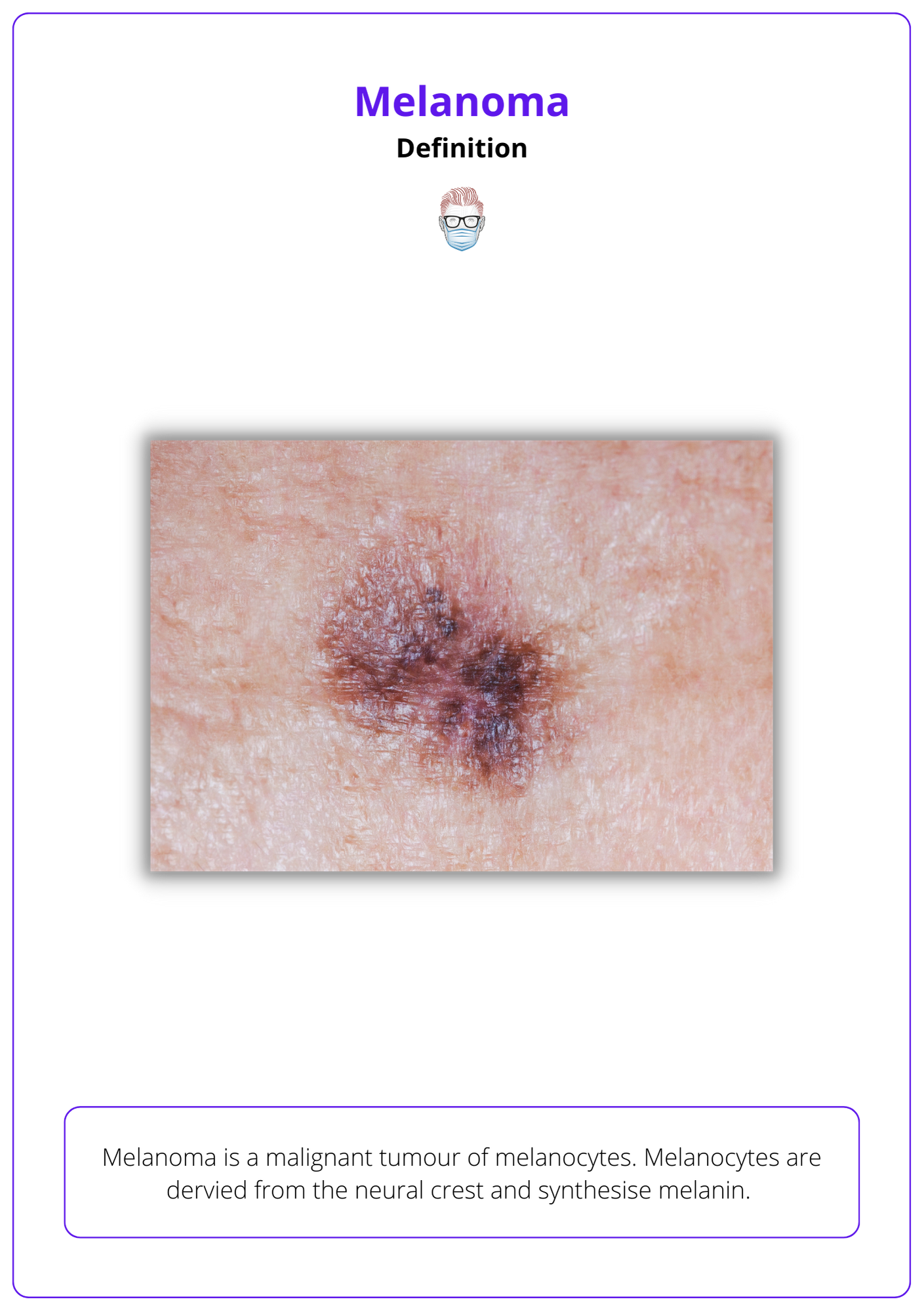
Definition of Melanoma
Melanoma is a malignant tumour of melanocytes. Melanocytes are derived from the neural crest and synthesise melanin.
Risk Factors for Melanoma
The risk of melanoma is dependent on the environment (radiation), genetic (BRAF) and patient-related factors (skin type and medical history).
Ultraviolet Radiation
The risk of melanoma increases with age from natural cumulative sun exposure. There is a strong correlation with the following:
- UVA (315-400nm): sunbeds, DNA damaged by oxygen radicals
- UVB (280-315nm): sunburn, absorbed by DNA, chromosomal damage
- UVC (200-280nm): filters by ozone
Fitzpatrick Type I + II
- Individuals with fair skin, hair, and eye colour
- Susceptibility to oncogenic UV damage in genes linked with pigmentation
Co-Morbidities
- Skin Lesions: previous melanoma, dysplastic lesions, giant CMNs
- Organ transplantation/immunosuppression
- Genetic syndromes: FAMM Syndrome
Diagnosis of Melanoma
Melanoma is a clinical suspicion confirmed with a histological diagnosis.
Clinical Suspicion
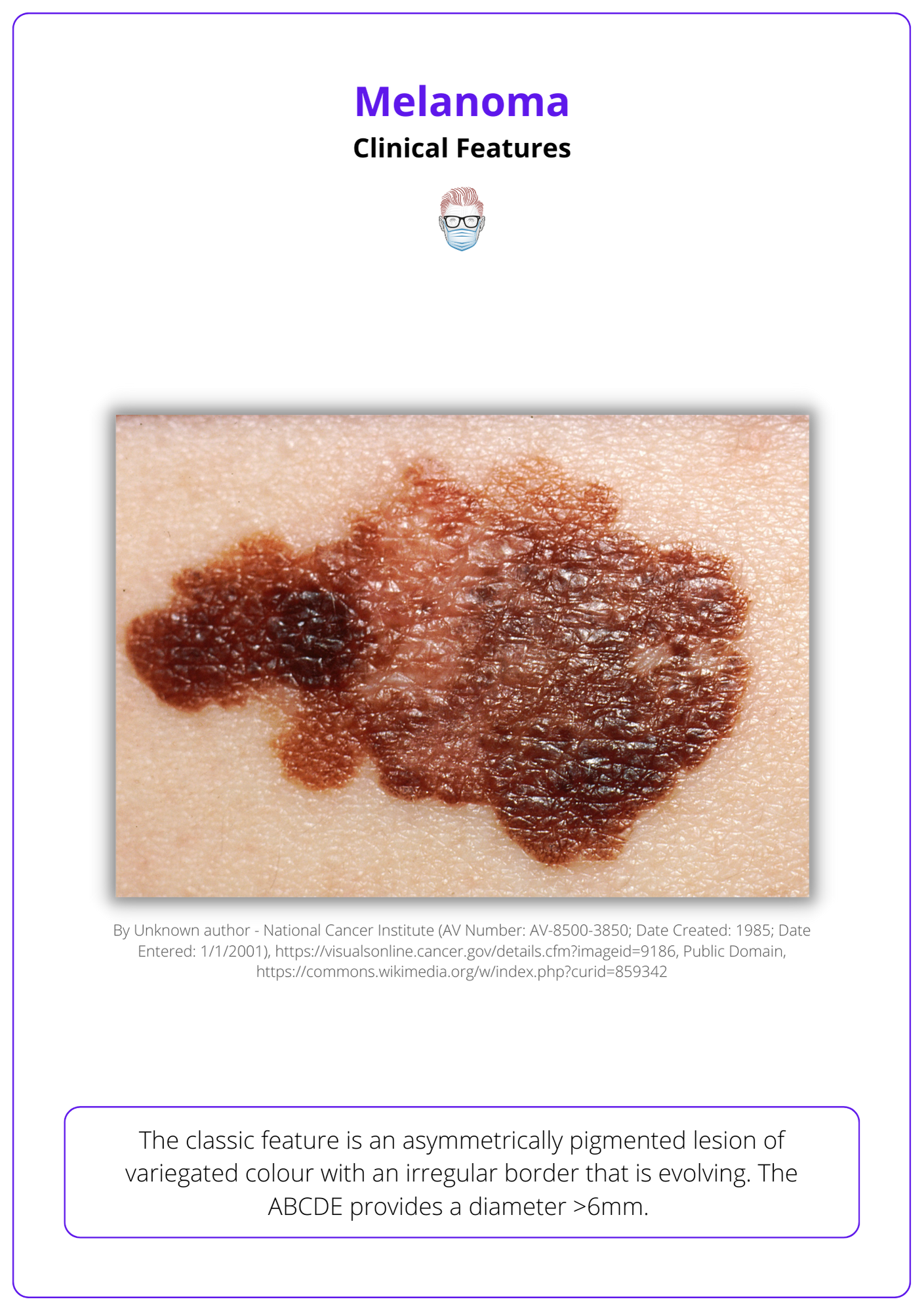
Cutaneous melanoma can be clinically suspected based on a history of lesion evolution and specific features on examination.
The classic feature is an asymmetrically pigmented lesion of variegated colour with an irregular border that is evolving. The ABCDE provides a diameter >6mm.
Below image illustrates the clinical features of melanoma.
Histological Diagnosis
Melanoma is a histological diagnosis. This is a key differentiator to basal cell and squamous cell carcinomas, which can be resected based on a confident clinical examination.
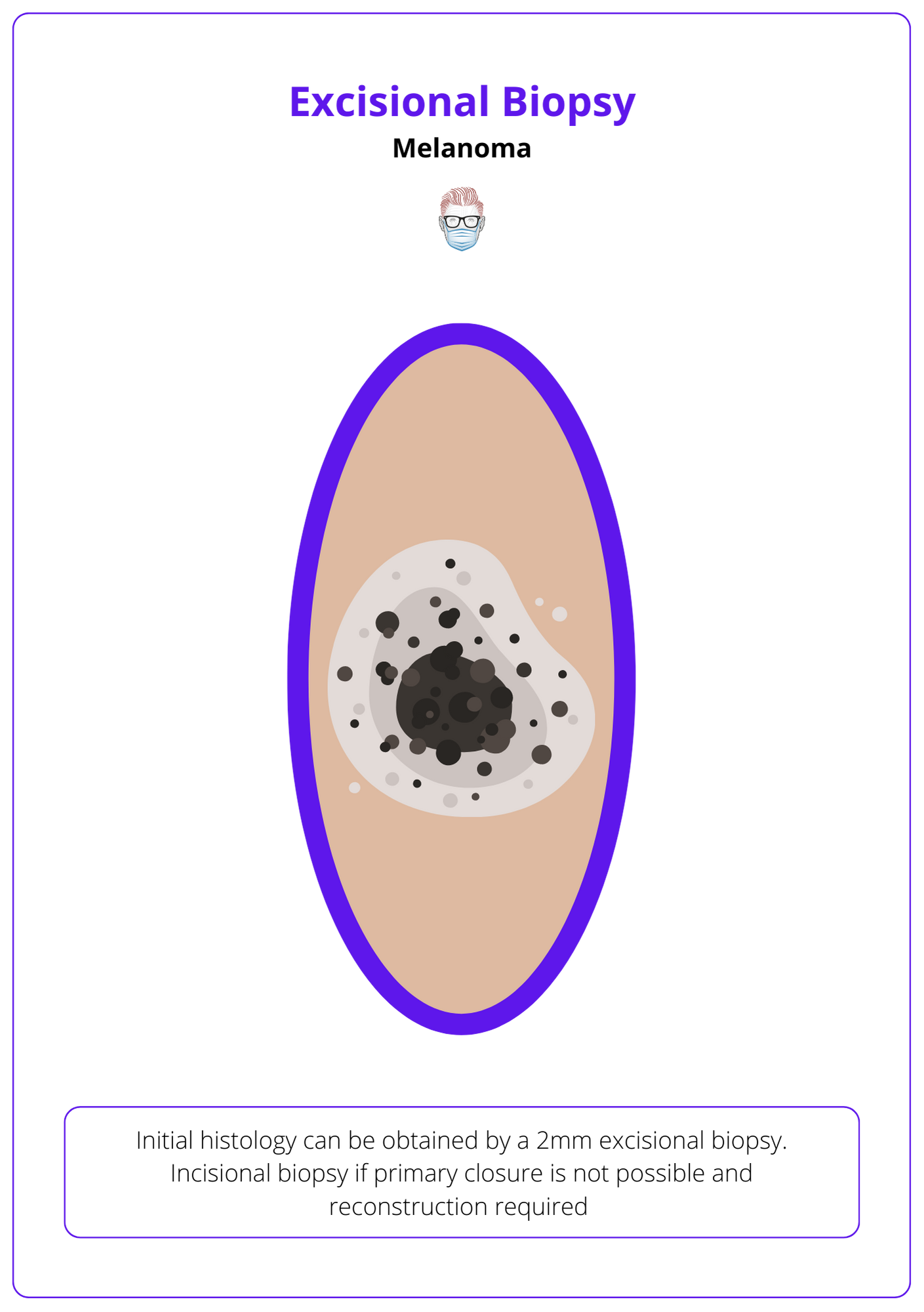
Initial histology can be obtained by:
- Excisional Biopsy with a 2mm margin and cuff of fat.
- Incisional biopsy if primary closure is not possible and reconstruction required.
- Punch Biopsy can be considered but can affect Breslow thickness.
- Shave or curettage is not recommended.
See image below for a visualization of the excisional biopsy of melanoma.
A melanoma histology report will provide the following information:
- Relevant clinical information
- Type of surgery: excisional, incisional, punch
- Location: most commonly on the trunk
- Macroscopic: vertical and depth dimensions
- Subtype: superficial, nodular, acral, lentigo, amelanocytic, desmoplastic
- Margins: margins excised if excisional biopsy performed
- Ulceration: full thickness loss of epidermis, a poor prognostic sign
- Mitosis: a marker for tumour activity, a poor prognostic sign
- Breslow Thickness: status granulosum to the deepest part of the lesion
- BRAF status: if requested, can decide suitability for immunotherapy
- Clarkes Level: level of dermal invasion
- Growth: radial or vertical, regression, LVI, PNI, microsatellites
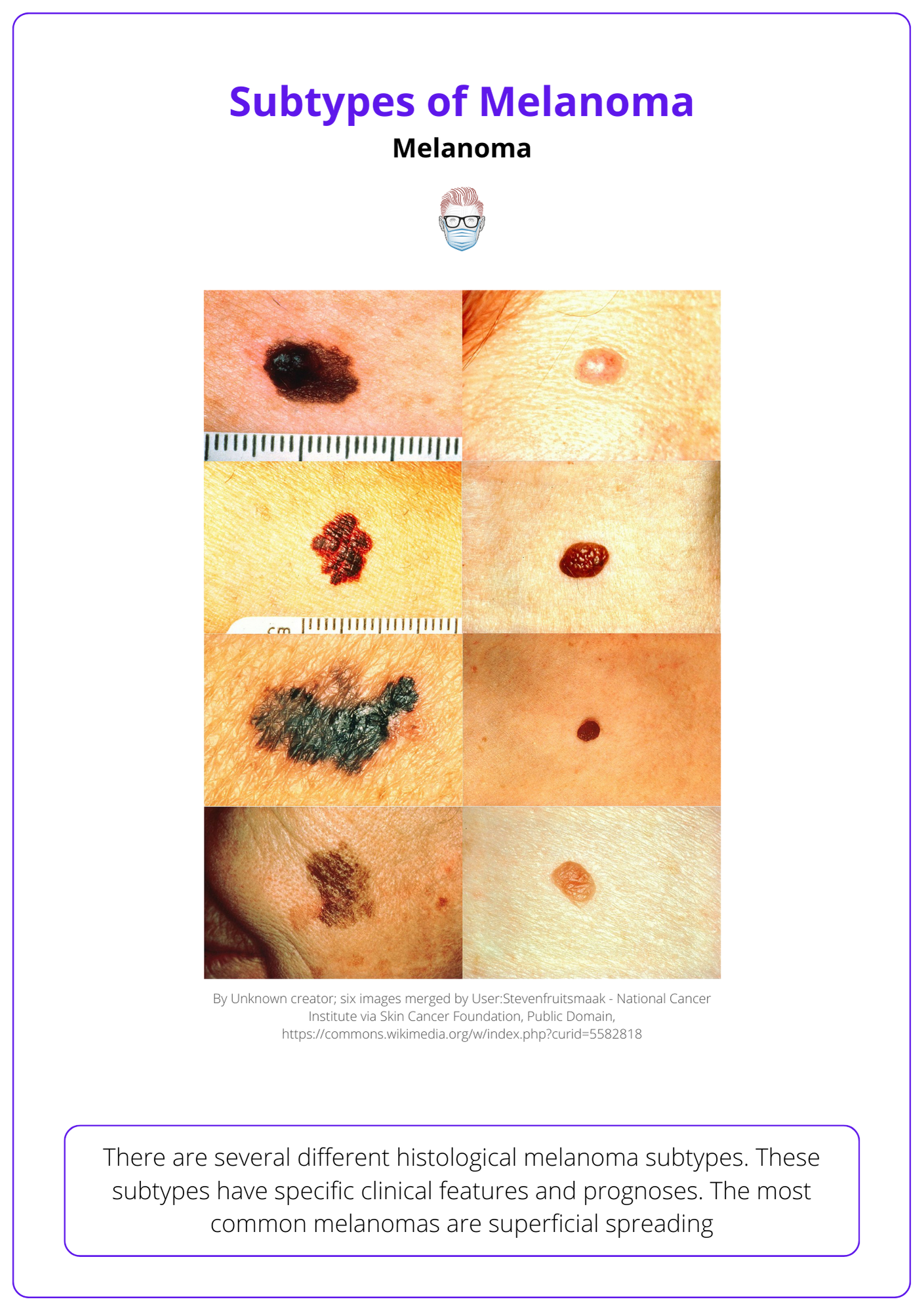
Types of Melanoma
The most common histological subtype is superficial spreading. Other subtypes include nodular, lentigo, acral, subungual, amelanocytic and desmoplastic.
There are several different histological melanoma subtypes. These subtypes have specific clinical features and prognoses. The most common melanomas are superficial spreading.
- Superficial spreading: radial then vertical growth phases.
- Nodular: is more aggressive because it lacks a radial growth phase.
- Lentigo Maligna Melanoma: arising from a lentigo maligna.
- Acral Lentiginous: usually occurs on palms, soles and nails.
- Amelanocytic: a melanoma without pigment.
- Desmoplastic: high local recurrence rates.
Below image visualizes different types of melanomas.
Wide Local Excision of Melanoma
The margins on a wide local excision are dependent on the Breslow thickness.
Wide Local Excision
The current guidelines for the excision of a histological-confirmed melanoma are based on the Breslow thickness of the lesion.
- Lentigo Maligna (in-situ): 0.5mm with a cuff of fat
- Breslow ≤ 1mm: 1cm peripheral margin, down to fascia deep margin
- Breslow 1-2mm: 1-2cm peripheral margin, down to fascia deep margin
- Breslow >2mm: 2 cm peripheral margin, down to fascia deep margin
See image below for layers of the Dermis and Epidermis.

Sentinel Lymph Node Biopsy
A sentinel node biopsy is indicated in melanoma ≥ pT2a or ≥ pT1b with high-risk features. Techniques include radioactive isotope, blue dye and indocyanine green.
A sentinel node is the first lymph node receiving lymphatic drainage from a specific area and is representative of that nodal basin1. The sentinel node has a role in prognosis, treatment pathways and staging. It should be performed at the time of wide local excision, if indicated.
Indications for SLNB
The primary role of sentinel node biopsy is changing from that of a prognostic indicator to one that now influences access to adjuvant therapy6.
- Primary Cutaneous Melanoma ≥ pT2a
- Primary Cutaneous Melanoma ≥ pT1b considered if LVI or Mitotis ≥ 2mm
It is not currently indicated for pT1a melanomas because the frequency of sentinel lymph node metastasis is <5% 8,9 compared to 4-12% for pT1b6 and 30% for BT>4mm8.10.
Effect of SLNB on Treatment
For those with a positive sentinel node, upstaging the disease allows earlier identification for adjuvant therapies. It also allows them to be categorized in a “high-risk follow-up group”7.
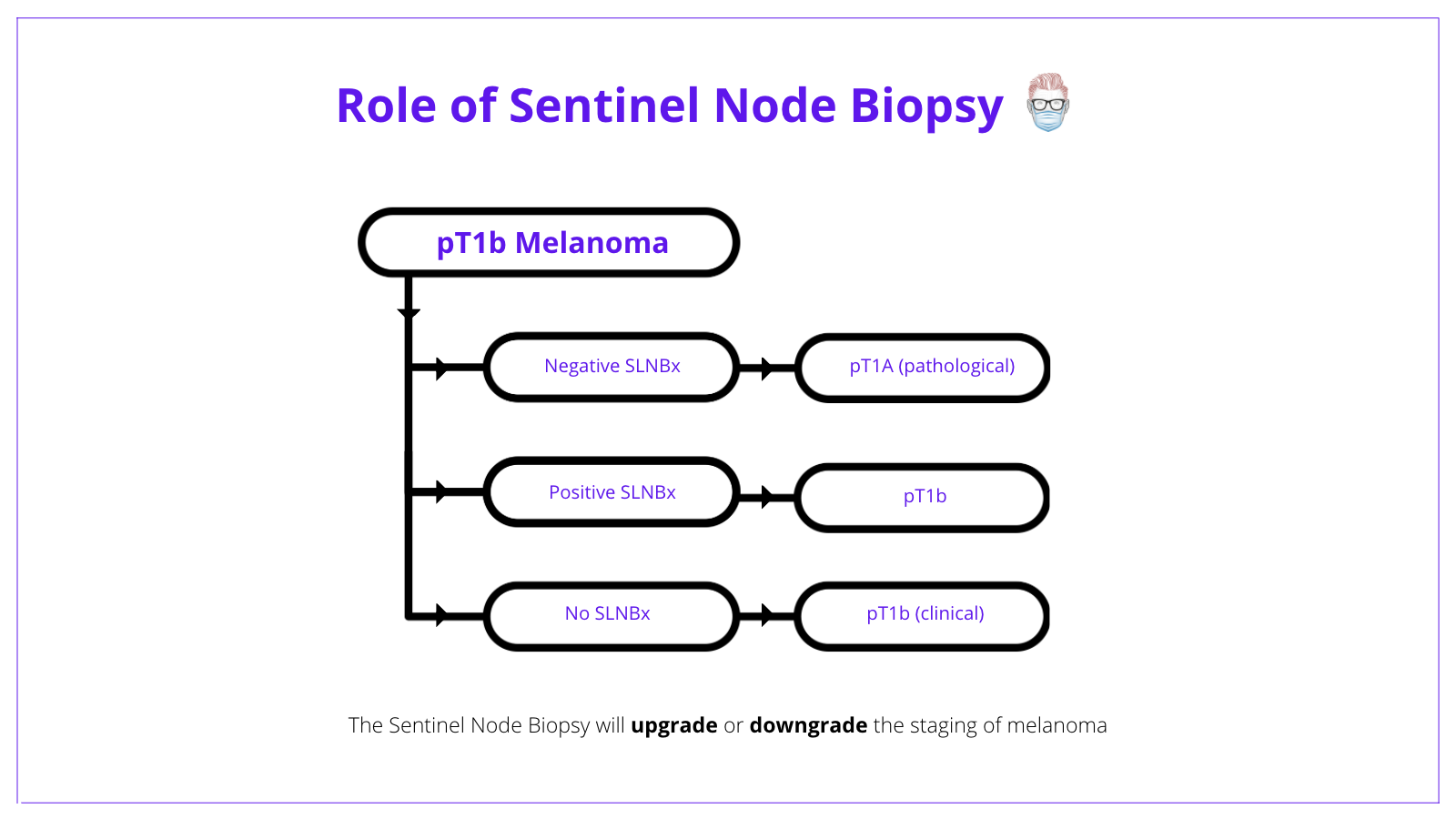
A patient with pT1b melanoma has 2 options:
- No SLNB = clinical Stage 1B with a 5-year survival of 97%3.
- Negative SLNB = pathological Stage 1A with a 5-year survival of 99%3.
- Positive SLNB = pathological Stage 1B.
The below image conceptualizes the role of sentinel node biopsy in treating melanoma.
Techniques
The 3 commonly used techniques for sentinel node biopsies are radioactive isotope, blue dye or indocyanine green.
Radioactive Isotope
- Intradermal injection of a radiotracer at the tumour site & lymphoscintigraphy.
- A handheld gamma probe guides dissection.
- Usually, Technetium-99m–labelled radiocolloids.
Blue Dyes (initially used by Morton et al)
- Blue dye alone sensitivity of 81% (Silvestri, 2019).
- Patent Blue V, isosulfan blue and methylene blue are commonly used.
- Anaphylaxis, obscured dissection, tattooing, interferes with pulse oximetry.
Indocyanine Green
- Indocyanine green is a fluorescent tricarbocyanine dye.
- Sensitivity: 91- 98%, with a specificity of 100% (Clloyd, 2014).
- It cannot be used in patients with iodine or shellfish allergies.
Positive Sentinel Node in Melanoma
Following a positive sentinel node, patients and doctors have three options: complete lymph node dissection, observation, and adjuvant therapies. These patients are now Stage III disease.
Completion Lymph Node Dissection in Melanoma
Complete lymph node dissection should not be routinely recommended for patients who have a positive sentinel node biopsy6. Consider this treatment option in patients who are high risk of regional relapse based on their sentinel node results:
- Extra-capsular spread
- 3 or more sentinel nodes
- Dewar Criteria12 (multifocal and extensive)
- Node-only recurrence having failed first-line systemic treatment
The MSLT-213 and DeCOG14 Trials reported on the outcome of patients with a positive sentinel node randomised into observation and surgery. Key results include:
- No survival advantage over radiological observation
- Increase risk of nodal recurrence in those patients with observation
Patients undergoing immediate lymph node dissection have:
- Increased rate of regional disease control13
- Prognostic information13
- No increase in melanoma-specific survival13
- An increased risk of morbidity in nodal dissection patients15
With head and neck melanomas, adjuvant therapy is preferred to neck dissection3.
Adjuvant Therapy in Melanoma
Discussed in more detail: Oncological Adjuvant Therapies in Melanoma
Current guidelines state adjuvant therapy should be available to Stage 3 patients.
Generally speaking, patients deemed at high risk with a positive sentinel node should be considered for melanoma adjuvant therapy. A lymph node dissection could be considered if they have a node-only recurrence post-adjuvant treatment.
Radiological Observation in Melanoma
There is currently no consensus for ideal imaging in melanoma7
Current options include:
- Ultrasound: MLST and DeCOG Trials but does have operator dependability5
- CT/PET: more sensitive to picking up recurrences5
- MRI is used in some UK centres6
Imaging surveillance will differ for those on adjuvant systemic therapy.
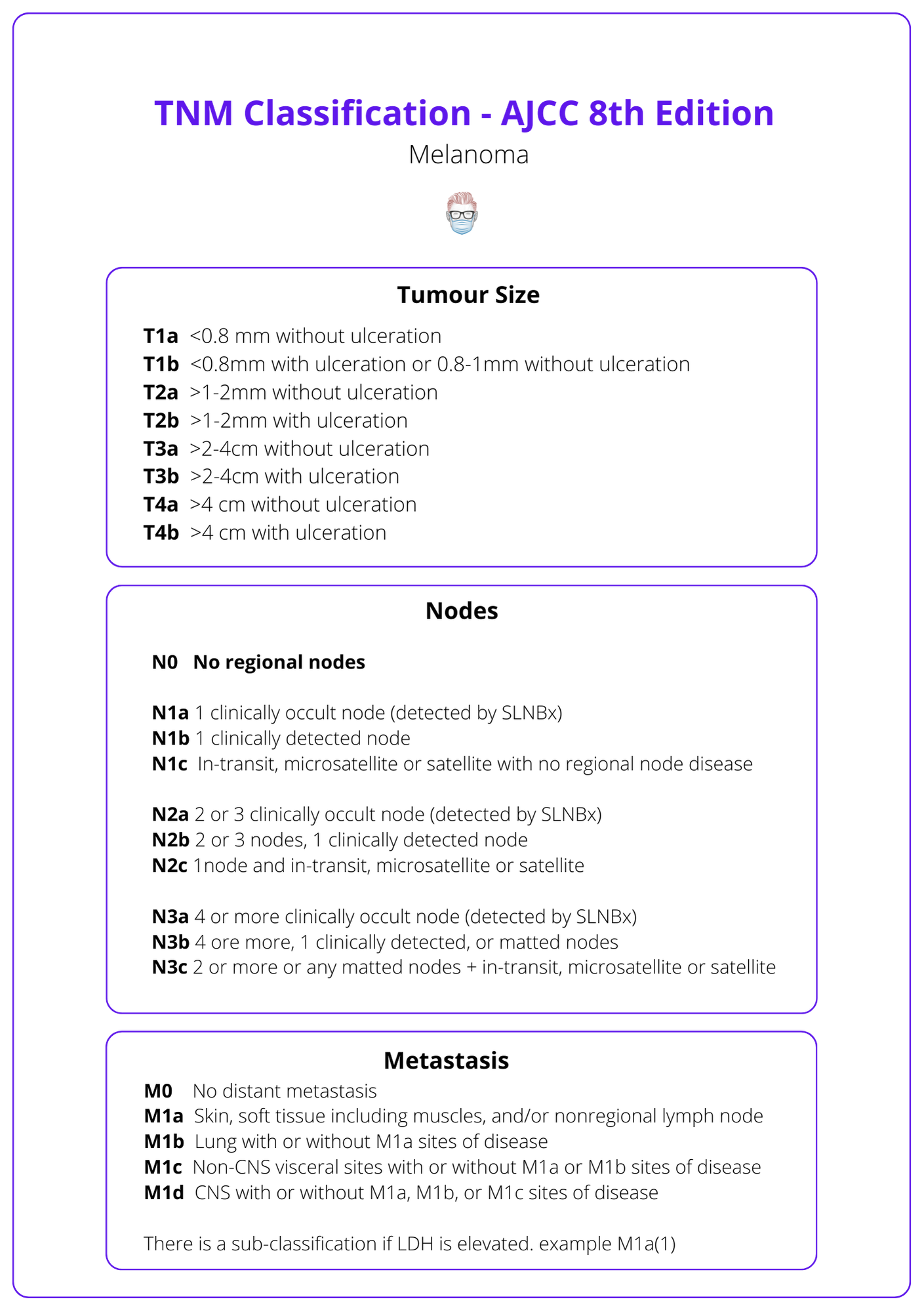
Staging Melanoma
Melanoma is classified and stages using the TNM system from the AJCC 8th Edition.
The image below details TNM Classification in staging melanoma.
AJCC Version 8 Updates
This article is written with the most recent version of AJCC and other publications, as referenced below. The following is a list of V8 updates3.
Histology Reports
- Tumour thickness: record to the nearest 0.1 mm, not 0.01 mm.
- Mitotic rate no longer a T category criterion (increased mitotic rate decreases melanoma-specific survival3).
Pathological Staging
- pT1a <0.8 mm without ulceration.
- pT1b 0.8‐1.0 mm with or without ulceration or <0.8 mm with ulceration.
- Pathological stage IA is revised to include T1b N0 M0 (formerly stage IB).
Metastatic disease
- N: “microscopic” and “macroscopic” is now clinically "occult" and "apparent".
- M1 subcategory designation for LDH (no longer upstages to M1c).
- M1d designation is added for central nervous system metastases.
Conclusion
1. Overview of Melanoma: Understood melanoma as a malignant tumor arising from melanocytes, with a complex interaction between genetic predispositions and environmental factors such as UV radiation.
2. Melanoma Risk Factors: Identified key risk factors for developing melanoma, which include exposure to UV radiation, genetic mutations (such as BRAF), and personal characteristics like skin type and medical history.
3. Diagnostic Procedures: Learned about the critical steps for diagnosing melanoma, emphasizing the necessity of histological examination to confirm clinical suspicions and detailed pathological analysis.
4. Surgical Management: Gained insights into the surgical management of melanoma, particularly the importance of wide local excision tailored by the Breslow thickness of the tumor and the role of sentinel lymph node biopsy in staging and treatment planning.
5. Other Management Strategies: Reviewed the management options available following a positive sentinel lymph node biopsy, ranging from complete lymph node dissection to observation and adjuvant therapies.
6. Staging and Prognostication: Familiarized with the TNM classification system for melanoma, which helps in staging the disease and guiding treatment decisions based on tumor depth and nodal involvement.
Further Reading
- Morton DL. Technical Details of Intraoperative Lymphatic Mapping for Early Stage Melanoma. Arch Surg. Published online April 1, 1992:392. doi:10.1001/archsurg.1992.01420040034005
- Dhakal S, Biswas T, Liesveld J, Friedberg J, Phillips G, Constine L. Patterns and timing of initial relapse in patients subsequently undergoing transplantation for Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys. 2009;75(1):188-192. doi:10.1016/j.ijrobp.2008.10.048
- Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA: A Cancer Journal for Clinicians. Published online October 13, 2017:472-492. doi:10.3322/caac.21409
- Lee JH, Park HS, Wei Q, Kim MN, Cho J-H. Difference of auditory brainstem responses by stimulating to round and oval window in animal experiments. Bioengineered. Published online September 30, 2016:8-13. doi:10.1080/21655979.2016.1226662
- Lee A, Droppelmann N, Panageas K, et al. Patterns and Timing of Initial Relapse in Pathologic Stage II Melanoma Patients. Ann Surg Oncol. 2017;24(4):939-946. doi:10.1245/s10434-016-5642-0
- Peach H, Board R, Cook M, et al. Current role of sentinel lymph node biopsy in the management of cutaneous melanoma: A UK consensus statement. J Plast Reconstr Aesthet Surg. 2020;73(1):36-42. doi:10.1016/j.bjps.2019.06.020
- National Collaborating Centre for Cancer (UK). Melanoma: Assessment and Management. Published online July 1, 2015.
- Kachare SD, Singla P, Vohra NA, Zervos EE, Wong JH, Fitzgerald TL. Sentinel lymph node biopsy is prognostic but not therapeutic for thick melanoma. Surgery. Published online September 2015:662-668. doi:10.1016/j.surg.2015.05.012
- Wat H, Senthilselvan A, Salopek TG. A retrospective, multicenter analysis of the predictive value of mitotic rate for sentinel lymph node (SLN) positivity in thin melanomas. Journal of the American Academy of Dermatology. Published online January 2016:94-101. doi:10.1016/j.jaad.2015.09.014
- Ribero S, Osella-Abate S, Sanlorenzo M, et al. Sentinel Lymph Node Biopsy in Thick-Melanoma Patients (N=350): What is Its Prognostic Role? Ann Surg Oncol. Published online November 12, 2014:1967-1973. doi:10.1245/s10434-014-4211-7
- Madu MF, Wouters MWJM, van Akkooi ACJ. Sentinel node biopsy in melanoma: Current controversies addressed. European Journal of Surgical Oncology (EJSO). Published online March 2017:517-533. doi:10.1016/j.ejso.2016.08.007
- Dewar D, Newell B, Green M, Topping A, Powell B, Cook M. The microanatomic location of metastatic melanoma in sentinel lymph nodes predicts nonsentinel lymph node involvement. J Clin Oncol. 2004;22(16):3345-3349. doi:10.1200/JCO.2004.12.177
- Faries MB, Thompson JF, Cochran AJ, et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N Engl J Med. Published online June 8, 2017:2211-2222. doi:10.1056/nejmoa1613210
- Leiter UM, Stadler R, Mauch C, et al. Final analysis of DECOG-SLT trial: Survival outcomes of complete lymph node dissection in melanoma patients with positive sentinel node. JCO. Published online May 20, 2018:9501-9501. doi:10.1200/jco.2018.36.15_suppl.9501
- Faries MB, Thompson JF, et al. The Impact on Morbidity and Length of Stay of Early Versus Delayed Complete Lymphadenectomy in Melanoma: Results of the Multicenter Selective Lymphadenectomy Trial (I). Ann Surg Oncol. Published online July 8, 2010:3324-3329. doi:10.1245/s10434-010-1203-0
- Leong SP, Thelmo MC, Kim RP, et al. Delayed harvesting of sentinel lymph nodes after previous wide local excision of extremity melanoma. Ann Surg Oncol. 2003;10:196–200.
- Leong WL, Ghazarian DM, McCready DR. Previous wide local excision of primary melanoma is not a contraindication for sentinel lymph node biopsy of the trunk and extremity. J Surg Oncol. 2003;82:143–146.
- Kelemen PR, Essner R, Foshag LJ, Morton DL. Lymphatic mapping and sentinel lymphadenectomy after wide local excision of primary melanoma. J Am Coll Surg. 1999;189:247–252.
- Karakousis CP, Grigoropoulos P. Sentinel node biopsy before and after wide excision of the primary melanoma. Ann Surg Oncol. 1999;6:785–789.
- McCready DR, Ghazarian DM, Hershkop MS, Walker JA, Ambus U, Quirt IC. Sentinel lymph-node biopsy after previous wide local excision for melanoma. Can J Surg. 2001;44:432–434.
- May MM, Lohse CM, Moore EJ, et al. Wide local excision prior to sentinel lymph node biopsy for primary melanoma of the head and neck. Int J Dermatol. 2019;58:1184–1190.


