Summary Card
Overview
Botulinum toxin (BoNT) from Clostridium botulinum temporarily relaxes muscles by inhibiting acetylcholine release, with serotypes A and B commonly used for therapeutic and aesthetic injections..
Mechanism of Action
BoNT-A cleaves SNARE proteins at the neuromuscular junction, blocking acetylcholine release, causing reversible muscle paralysis typically lasting 3–6 months.
Types
BoNT-A brands (e.g., Botox®, Dysport®, Xeomin®, Jeuveau®) and BoNT-B (MyoBloc®) vary in potency, formulation, and clinical applications.
Therapeutic Applications
Indications include migraines, cervical dystonia, hyperhidrosis, and off-label uses such as bruxism, tremors, and cosmetic wrinkle reduction.
Injection Technique
Dilute (about 2–2.5 mL/100 U) and inject precisely into target muscles, tailoring dose and placement to individual anatomical needs.
Contraindications and Adverse Effects
Avoid if infection, hypersensitivity, neuromuscular disorders, or pregnancy are present; potential adverse effects include local pain, bruising, and rare systemic complications.
Primary Contributor: Dr Benedetta Agnelli, Educational Fellow.
Overview of Botulinum Toxin
Botulinum toxin (BoNT) from Clostridium botulinum temporarily relaxes muscles by inhibiting acetylcholine release, with serotypes A and B commonly used for therapeutic and aesthetic injections..
Botulinum toxin, also known as Botox or BTX, is produced by a Gram-positive, anaerobic bacterium - Clostridium botulinum. It works by inhibiting ACh release, leading to temporary muscle paralysis. Clinically, it is used to treat spasticity, dystonia, hyperhidrosis, and for aesthetic enhancements.
There are several subtypes of Botox:
- Type A & B: Most commonly used in clinical practice. Type A is preferred for its high potency and long duration of action, while Type B serves as an alternative for patients resistant to Type A.
- Types E & F: Rarely cause human disease and have limited clinical application.
Botulinum toxin holds FDA approval for various medical and cosmetic indications, with numerous additional off-label uses
Historical Highlights
- 1870: Dr. Muller introduces the term "botulism."
- 1970s: Dr. Alan Scott uses BoNT-A for strabismus.
- 1980s: Dr. Jean Carruthers observes wrinkle reduction in patients treated for blepharospasm, recognizing BoNT-A’s aesthetic potential.
- 1992: A landmark publication highlights its cosmetic benefits.
- 2002: The FDA approves BoNT-A for glabellar lines.
Below, you can see the first publication on Botox.

In 2023, approximately 8.9 million BoNT-A procedures were performed worldwide—surpassing the combined total of liposuction, breast augmentation, rhinoplasty, abdominoplasty, and blepharoplasty procedures
Mechanism of Action of Botulinum Toxin
BoNT-A cleaves SNARE proteins at the neuromuscular junction, blocking acetylcholine release, causing reversible muscle paralysis typically lasting 3–6 months.
The primary action of Botulinum neurotoxin (BoNT) is the inhibition of ACh release at neuromuscular junctions results in reduced muscle contraction, leading to temporary flaccid paralysis. It does not impact adrenergic nerve endings, maintaining selective effects on cholinergic synapses.
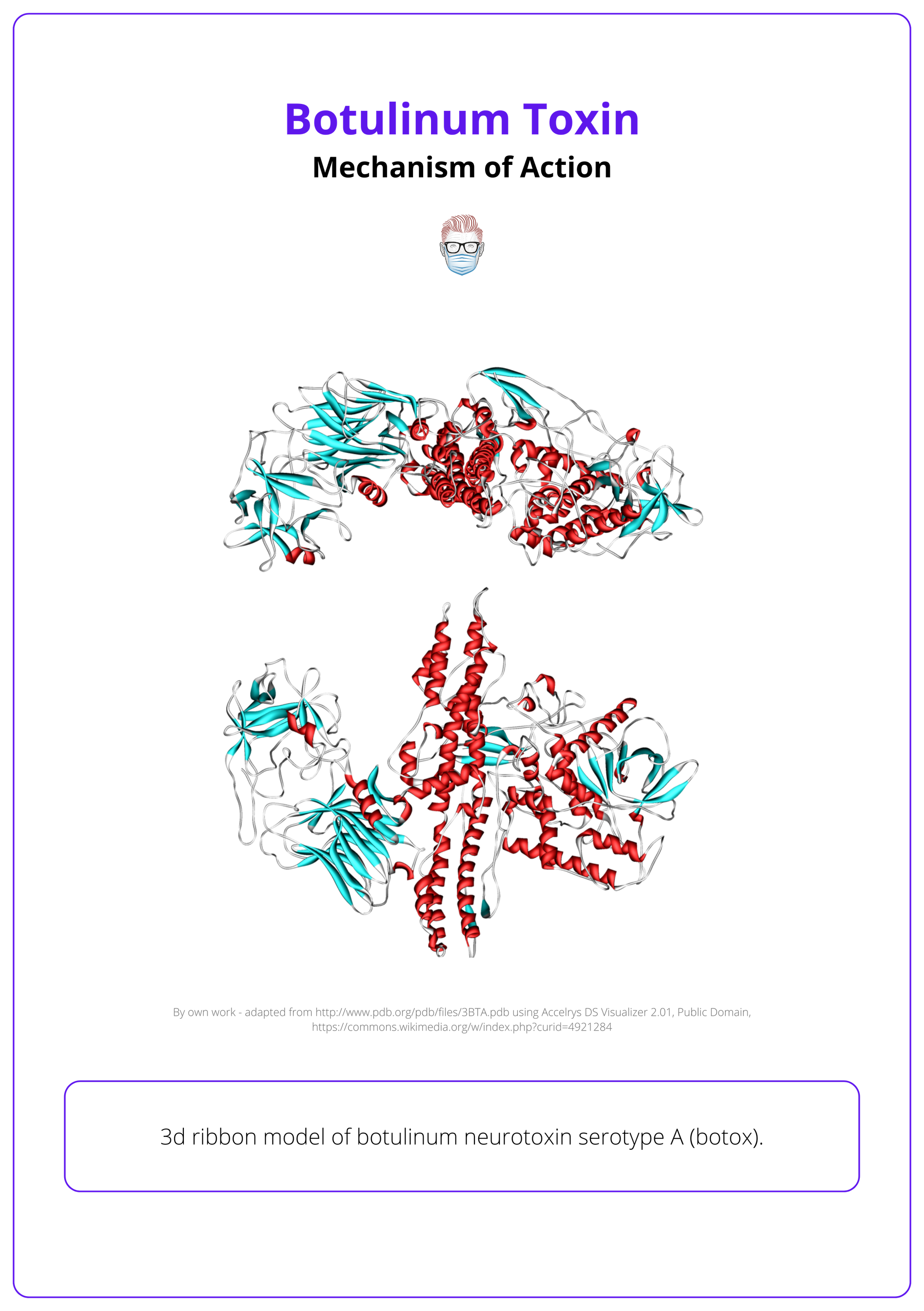
Structure
Botulinum neurotoxin is a 150 kDa polypeptide, structurally and functionally divided into two main chains.
- Heavy Chain (100 kDa): Facilitates neuronal targeting into presynaptic cholinergic nerve terminals. Binds specifically to ganglioside receptors and protein components of the presynaptic membrane.
- Light Chain (50 kDa): Acts as a zinc-dependent endopeptidase (protease). Responsible for cleaving essential SNARE (Soluble NSF Attachment Protein Receptor) proteins, disrupting neurotransmitter release.
Mechanism of Action
- Binding: BoNT selectively attaches to presynaptic cholinergic nerve terminals via specific surface receptors, ensuring targeted delivery to motor neurons.
- Internalization: The toxin-receptor complex is internalized through receptor-mediated endocytosis into the nerve terminal.
- Cleavage of SNARE Proteins: The light chain exits the endosome into the cytoplasm and enzymatically cleaves SNARE proteins critical for the docking and fusion of acetylcholine (ACh)-containing vesicles with the presynaptic membrane.
The image below illustrates the mechanism of action of Botox.
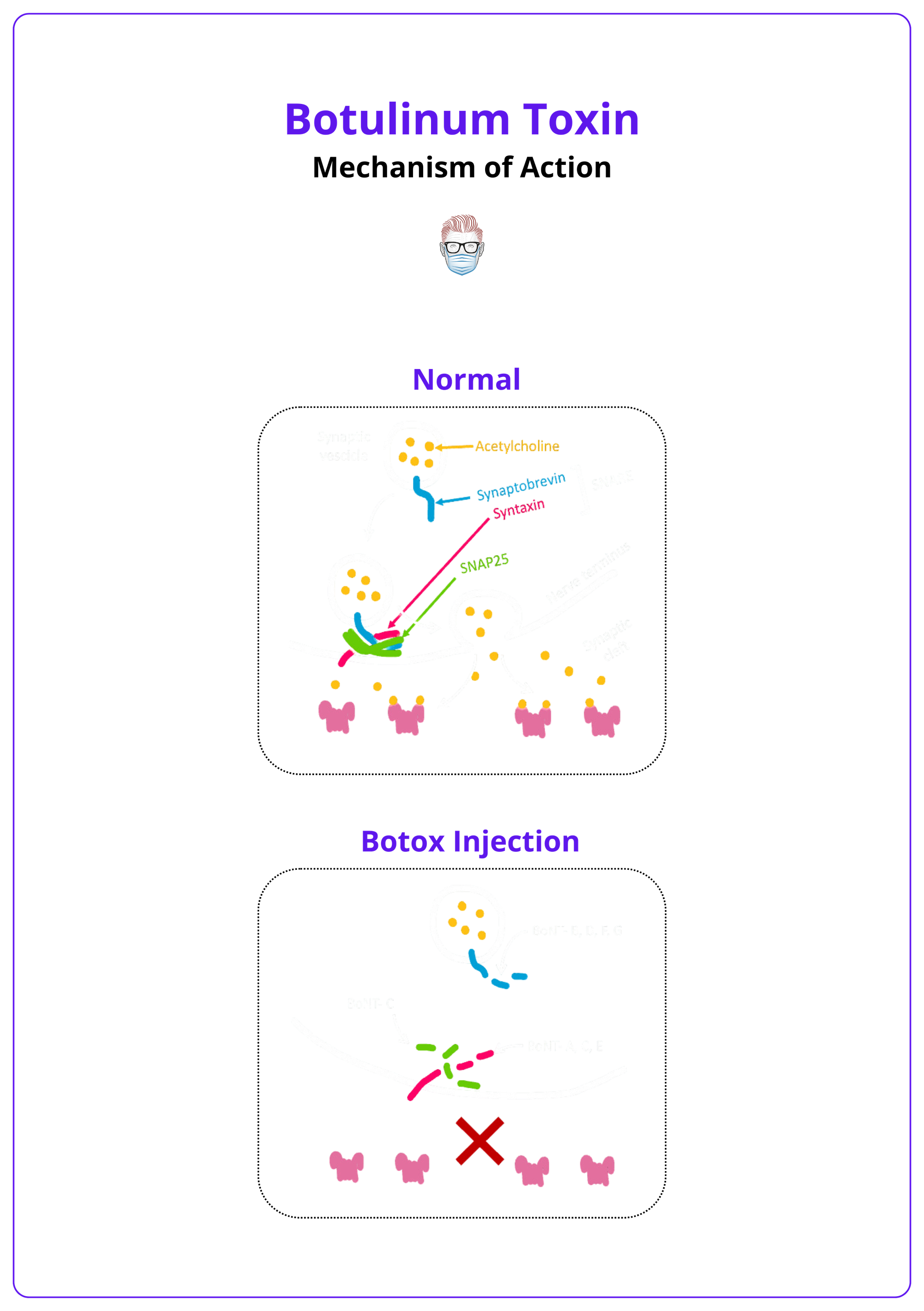
BoNT-A/E cleave SNAP-25, BoNT-B/D/F/G cut synaptobrevin, and BoNT-C targets both SNAP-25 and syntaxin.
Timeline
- Clinical onset occurs within 3–5 days post-administration.
- Maximal effect is observed by 10 days.
- Recovery over 3–6 months occurs through axonal sprouting and formation of new neuromuscular junctions
Duration varies based on individual metabolism and injection site.
Types of Botulinum Toxin
BoNT-A brands (e.g., Botox®, Dysport®, Xeomin®, Jeuveau®) and BoNT-B (MyoBloc®) vary in potency, formulation, and clinical applications.
Several botulinum toxin formulations are available, each with unique properties, production methods, and clinical uses. Importantly: Botulinum toxin units are not interchangeable between brands,
Key preparations are noted in the table below.
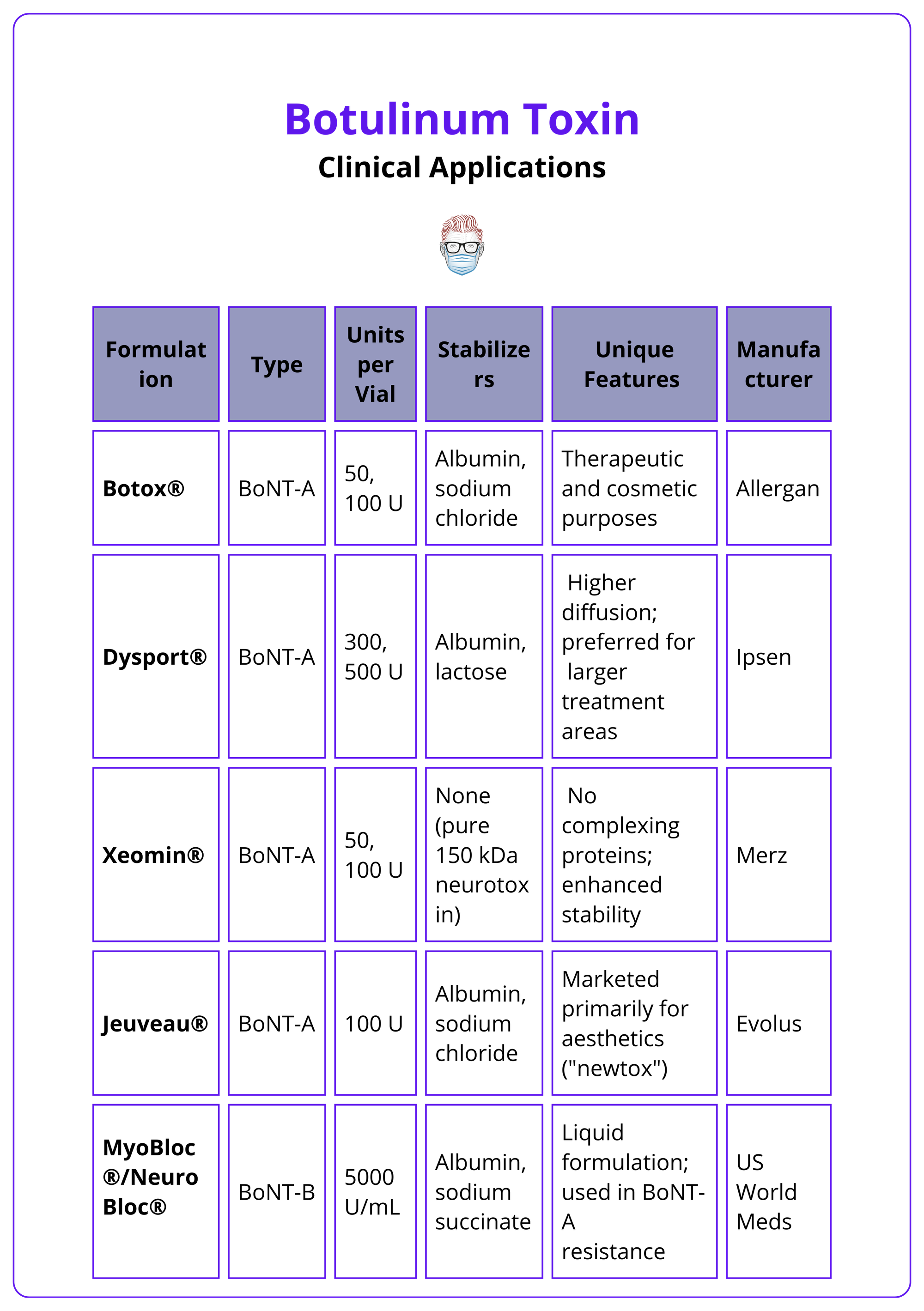
Botox should be stored at 2–8°C, and reconstituted toxin must be used within 4 hours if kept at room temperature or within 24 hours if refrigerated. Xeomin® offers superior stability at room temperature.
Therapeutic Applications of Botulinum Toxin
Indications include migraines, cervical dystonia, hyperhidrosis, and off-label uses such as bruxism, tremors, and cosmetic wrinkle reduction.
BoNT-A addresses both medical and aesthetic needs, with FDA-approved uses like treating chronic migraines and hyperhidrosis. Off-label, it aids in managing pain, movement disorders, and scars.
Cosmetic Applications
From a cosmetic perspective, Botulinum toxin’s ability to provide localized, long-lasting neuromuscular inhibition with minimal invasiveness has made it a cornerstone treatment. It plays a critical role:
- Facial Rhytids: Treatment of facial muscles of expression and mastication. •
- Hypertrophic Scars: Reduce tension & improve scar remodeling.
- Migraine: Diagnostic & therapeutic adjunct before surgical interventions.
- Vasospastic: Raynaud’s phenomenon & scleroderma-associated pathology.
Other Applications
Botulinum toxin is widely used in FDA-approved treatments and off-label applications, highlighting its neuromodulatory versatility.
The toxin’s efficacy has led to formal FDA-Approved uses for several medical conditions:
- Neurologic: Chronic migraines, cervical dystonia, focal spasticity.
- Hyperhidrosis: Axillary hyperhidrosis refractory to topical treatments.
- Urologic: Overactive bladder & urinary incontinence from detrusor overactivity
Expanding beyond approved indications, BoNT is utilized for various Off-Label neuromodulatory applications:
- Dystonia/Bruxism: Reducing involuntary movements * clenching.
- Tremors: Including essential and parkinsonian rest tremors.
- Pain: Chronic pain, eg. myofascial pain syndrome & neuropathic pain.
Injection Technique of Botulinum Toxin
Dilute (about 2–2.5 mL/100 U) and inject precisely into target muscles, tailoring dose and placement to individual anatomical needs.
Preparation & Dilution
Proper preparation ensures accurate dilution, toxin integrity, and optimal results. Importantly 1 unit = 0.02 mL when 50 U is diluted with 1 mL of sterile, preservative-free saline.
- Cosmetic applications: 2 mL/100 U or 2.5 mL/100 U based on area/ effect.
- Hyperhidrosis: Higher dilutions (4–5 mL/100 U) to cover larger areas.
Gently inject the sterile, preservative-free saline as a diluent to avoid frothing; do not shake the vial vigorously.
Procedure
Tailoring the approach to the patient’s anatomy and goals is crucial for natural and effective outcomes.
- Patient Communication: Discuss the procedure and align expectations. Obtain informed consent.
- Needle Selection: Use a fine-gauge needle (30–32G) for comfort and accuracy.
- Injection Site Planning: Sterilize the area and mark sites based on functional anatomy and treatment goals.
- Anatomical Assessment: Observe the patient’s expressions in repose and animation to ensure natural results. This dual analysis helps deliver results that maintain natural facial expressions while effectively reducing wrinkles, avoiding an undesired "frozen" appearance.
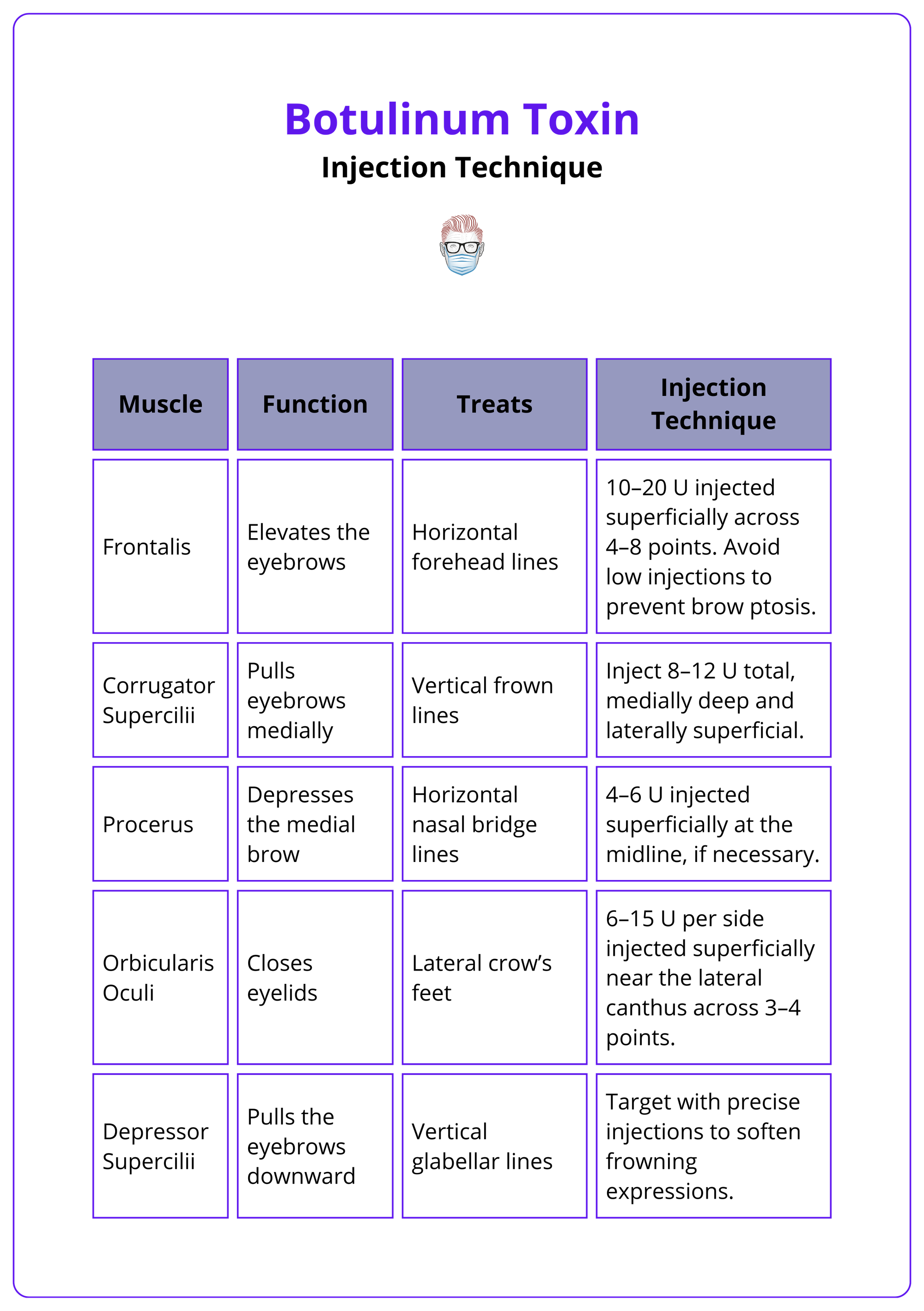
Frontal Region
Targeting specific anatomical regions ensures precise treatment outcomes, balancing efficacy with the preservation of natural expressions.
- Frontalis: Elevates the eyebrows; treats horizontal forehead lines with 10–20 U injected superficially across 4–8 points. Avoid low injections to prevent brow ptosis.
- Corrugator Supercilii: Pulls eyebrows medially, causing vertical frown lines; inject 8–12 U total, medially deep and laterally superficial, to address these lines effectively.
- Procerus: Depresses the medial brow, forming horizontal nasal bridge lines; target these lines with 4–6 U injected superficially at the midline, if necessary.
- Orbicularis Oculi: Closes eyelids and contributes to lateral crow’s feet; smooth crow’s feet with 6–15 U per side injected superficially near the lateral canthus across 3–4 points.
- Depressor Supercilii: Pulls the eyebrows downward, enhancing vertical glabellar lines; softens frowning expressions by targeting this muscle with precise injections.
The table below summarizes the above treatment techniques.
Glabeller Regiojn
The glabellar complex demonstrates distinct contraction patterns, requiring tailored injection approaches to address specific muscle dynamics:
- V-Pattern: Strong approximation and depression of the medial eyebrows; treat with 7-point injections focusing on the corrugator and procerus using higher doses.
- U-Pattern: Mild approximation and depression with arched brows at rest; use 5 classic injection points targeting the corrugator and procerus.
- Omega Pattern: Medial brow elevation with lateral depression, forming an "Ω" shape; target the medial frontalis and corrugator with precise injections.
- Converging Arrows: Horizontal approximation without notable lifting or depression; focus injections on the corrugator and orbicularis muscles.
- Inverted Omega: Predominant lowering of the brows, forming an inverted "Ω"; emphasize injections in the procerus and depressor supercilii muscles.
Maintain at least 1,5 cm above the supraorbital rim for frontalis injections to avoid ptosis.
Nasolabial and Perioral Region
Targeting specific muscles in the nasolabial and perioral region ensures effective treatment of bunny lines, perioral wrinkles, and downward mouth corners while preserving natural movement.
- Nasalis: Treats bunny lines with 4–6 U injected superficially into the muscle belly, avoiding adjacent muscles.
- Depressor Anguli Oris (DAO): Addresses downward mouth corners with 3–5 U per side injected deeply at the lateral jawline.
- Orbicularis Oris: Targets perioral wrinkles with 2–4 U per side injected superficially along the vermillion border. Avoid midline injections to maintain lip mobility.
Over-injection in the perioral area can lead to speech and eating difficulties.
Masseter and Jawline Region
Focused treatment of the masseter muscle enhances jawline contour and alleviates temporomandibular joint (TMJ) pain. It treats masseter hypertrophy for jawline contouring and TMJ pain relief.
- Anatomy: Composed of superficial and deep layers, the masseter originates from the zygomatic arch and inserts on the mandible. Contraction causes jaw clenching and contributes to a square jawline.
- Injection Technique: Inject 20–30 U per side deeply near the mandibular angle, dividing the dose into 3–5 points. Ensure deep placement and avoid the risorius muscle to prevent an asymmetrical smile.
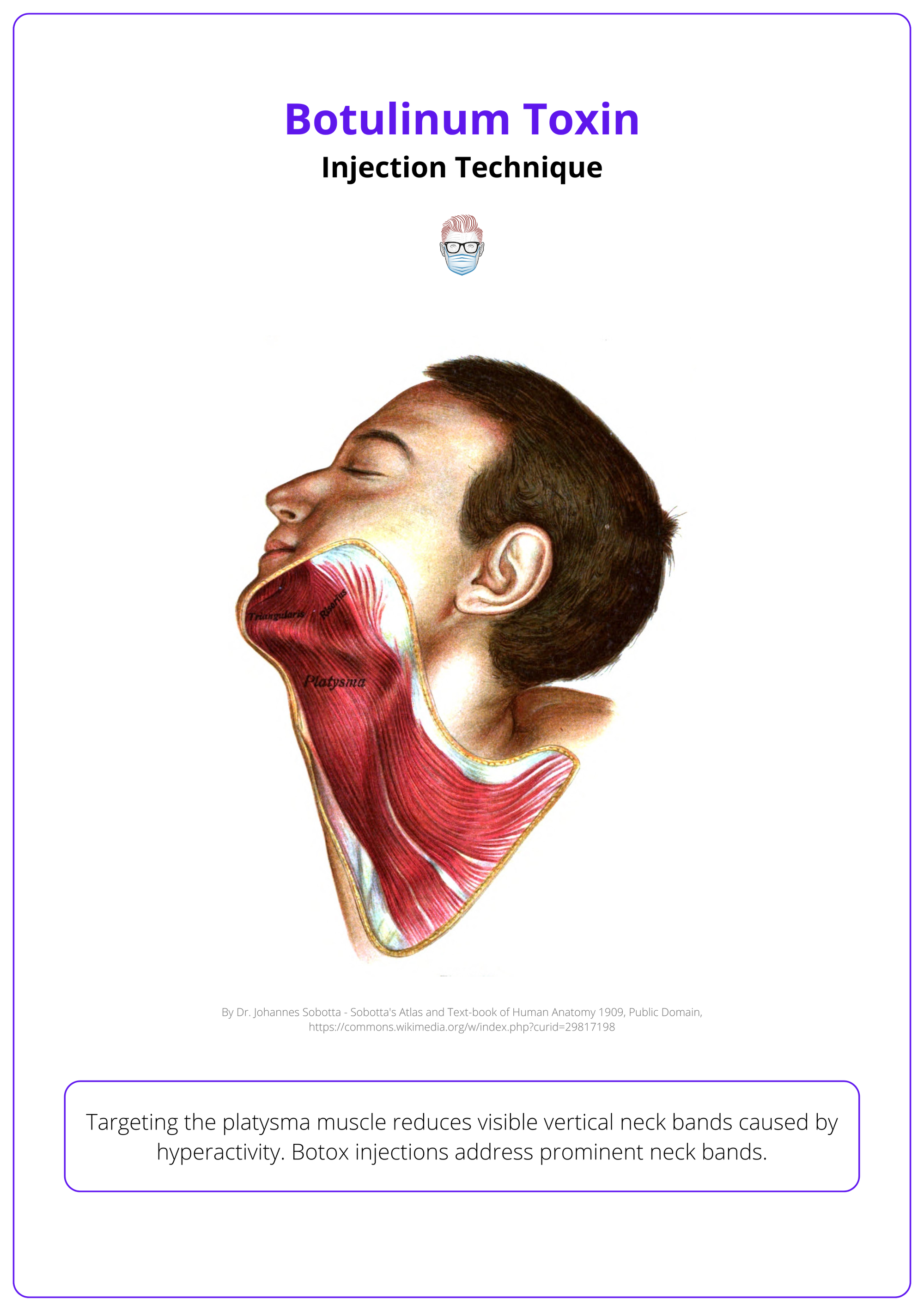
Platysmal Bands (Neck Region)
Targeting the platysma muscle reduces visible vertical neck bands caused by hyperactivity. Botox injections address prominent neck bands.
- Anatomy: Originates from the chest and inserts on the mandible, creating vertical bands when contracted.
- Injection Technique: Pinch the bands and inject 4–6 U directly into each band using a pinch-and-release technique. Avoid deep structures to prevent dysphagia.
Below is an illustration of the anatomy of platysmal bands.
Contraindications and Adverse Effects
Avoid if infection, hypersensitivity, neuromuscular disorders, or pregnancy are present; potential adverse effects include local pain, bruising, and rare systemic complications.
Proper use of botulinum toxin requires careful attention to contraindications such as neuromuscular disorders and allergies, along with vigilant monitoring for localized or systemic adverse effects to prevent complications.
Contraindications
Botulinum toxin should not be used in the following situations to prevent adverse outcomes:
- Infection at the Injection Site: Active infection in the proposed treatment area.
- Hypersensitivity: Allergic reactions to botulinum toxin preparations or excipients.
- Neuromuscular Disorders: Conditions like myasthenia gravis or Lambert-Eaton syndrome, which increase sensitivity to the toxin.
- Pregnancy and Lactation: The safety of botulinum toxin during pregnancy or breastfeeding is unproven, so it should be avoided.
Additional Considerations:
- Avoid in patients taking aminoglycosides or neuromuscular blockers, which may amplify the toxin's effects.
- For certain formulations (e.g., Dysport), lactose sensitivity or cow’s milk protein allergy is a contraindication due to trace ingredients.
Adverse Effects
While botulinum toxin is generally safe, some adverse effects may occur:
Local Effects
- Pain, erythema, or bruising at the injection site.
- Edema or transient headache.
- Local muscle weakness or asymmetry due to unintended diffusion.
Topical α-adrenergic agents can counter eyelid ptosis caused by inadvertent levator palpebrae superioris injection.
Systemic Effects (Rare)
- Botulism-like symptoms, including dysphagia, dysphonia, or generalized muscle weakness.
- Respiratory complications, particularly in patients with neuromuscular disorders or with high therapeutic doses.
Warnings on Toxin Spread
Post-marketing reports indicate that botulinum toxin effects may extend beyond the injection site, especially with high doses used therapeutically:
- Ocular Effects: Diplopia, blurred vision, ptosis.
- Oropharyngeal and Respiratory Symptoms: Dysphagia, dysphonia, and respiratory difficulties.
These effects may emerge hours to weeks after injection, emphasizing the need for careful patient selection and appropriate dosing.
Conclusion
1. Overview: Botulinum toxin (BoNT), a neurotoxin derived from Clostridium botulinum, inhibits acetylcholine release at neuromuscular junctions, causing temporary muscle paralysis.
2. Mechanism of Action: BoNT cleaves SNARE proteins required for acetylcholine release, preventing muscle contraction. Effects peak within 10 days and last 3–6 months.
3. Applications: FDA-approved uses include chronic migraines, cervical dystonia, hyperhidrosis, and overactive bladder. Off-label uses extend to facial aesthetics, scar remodeling, myofascial pain, and Raynaud’s phenomenon.
4. Injection Techniques: Successful outcomes depend on proper dilution, anatomy knowledge, and precise injection planning.
5. Contraindications and Adverse Effects: BoNT is contraindicated in active infections, hypersensitivity, and neuromuscular disorders.
Further Reading
- de Almeida AR, da Costa Marques ER, Banegas R, Kadunc BV. Glabellar contraction patterns: a tool to optimize botulinum toxin treatment. Dermatol Surg. 2012 Sep;38(9):1506-15. doi: 10.1111/j.1524-4725.2012.02505.x. Epub 2012 Jul 16. PMID: 22804914.
- Muñoz-Gonzalez C, Fakih-Gomez N. Resolving the Controversy Surrounding the Function of the Corrugator Supercilii Muscle. Aesthetic Plast Surg. 2024 Oct 24. doi: 10.1007/s00266- 024-04454-8. Epub ahead of print. PMID: 39448446.
- Cotofana S, Freytag DL, Frank K, Sattler S, Landau M, Pavicic T, Fabi S, Lachman N, Hernandez CA, Green JB. The Bidirectional Movement of the Frontalis Muscle: Introducing the Line of Convergence and Its Potential Clinical Relevance. Plast Reconstr Surg. 2020 May;145(5):1155-1162. doi: 10.1097/PRS.0000000000006756. PMID: 32332530.
- Borba A, Matayoshi S, Rodrigues M. Avoiding Complications on the Upper Face Treatment With Botulinum Toxin: A Practical Guide. Aesthetic Plast Surg. 2022 Feb;46(1):385-394. doi: 10.1007/s00266-021-02483-1. Epub 2021 Aug 2. PMID: 34341857; PMCID: PMC8328485.
- Kaplan JB. Consideration of Muscle Depth for Botulinum Toxin Injections: A Three Dimensional Approach. Plast Surg Nurs. 2017 Jan/Mar;37(1):32-38. doi:10.1097/PSN.0000000000000172. PMID: 28244963.
- Hsu TS, Dover JS, Arndt KA. Effect of volume and concentration on the diffusion of botulinum exotoxin A. Arch Dermatol. 2004 Nov;140(11):1351-4. doi: 10.1001/archderm.140.11.1351. PMID: 15545544.


