Summary Card
Overview
A rare T-cell lymphoma linked to textured breast implants, characterised by delayed-onset seroma, swelling, or masses.
Pathogenesis
Chronic inflammation from textured implants, bacterial biofilm formation, and genetic predisposition drive disease development.
Risk Factors
Associated with macrotextured implants, with most cases presenting 8–10 years post-implantation.
Diagnosis
Diagnosis relies on clinical evaluation, imaging, and pathological confirmation via cytology, flow cytometry, and CD30 immunohistochemistry.
Staging
The TNM staging system for solid tumors is preferred over traditional lymphoma staging methods for BIA-ALCL.
Management
Complete en bloc resection, including implant and capsule removal, is essential. Advanced cases may require chemotherapy (e.g., brentuximab vedotin) or radiotherapy.
Primary Contributor: Dr Hatan Mortada.
Overview of BIA-ALCL
BIA-ALCL is a rare, distinct type of T-cell lymphoma associated primarily with textured breast implants, characterized by delayed seroma formation and favorable prognosis when treated early.
Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) is a rare type of T-cell lymphoma linked to textured breast implants.
First described by Keech and Creech in 1997, it predominantly arises in the fibrous capsule or fluid surrounding the implant. This original publication can be seen below.
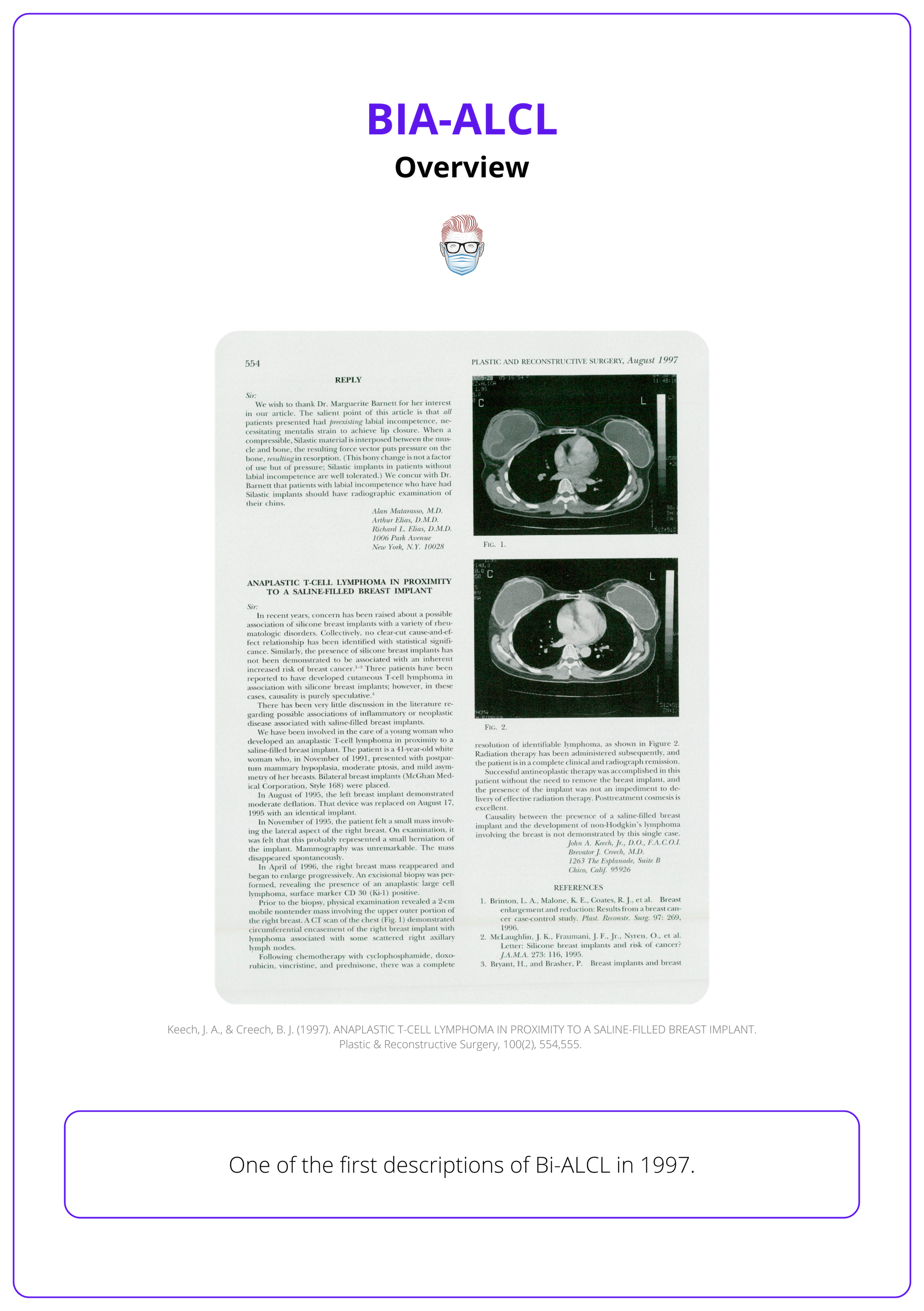
In 2016, the World Health Organization (WHO) classified BIA-ALCL as a distinct disease due to its clinical significance and increasing recognition.
Pathogenesis of BIA-ALCL
Chronic inflammation, bacterial biofilm, and genetic predisposition collectively contribute to BIA-ALCL development.
The development of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) is driven by a complex interplay of chronic inflammation, bacterial contamination, genetic predisposition, and environmental factors in susceptible individuals.
- Chronic Inflammation: Textured implants incite prolonged immune activation through friction and particulate shedding, driving dysregulated JAK/STAT signaling. Persistent immune responses create a microenvironment conducive to T-cell transformation.
- Bacterial Biofilm: Textured implants harbor higher bacterial loads, leading to chronic antigenic stimulation and T-cell monoclonality. This prolonged stimulation drives the development of monoclonal CD30+ T-cells, a hallmark of BIA-ALCL.
- Genetic Susceptibility: Mutations in regulatory genes (e.g., STAT3, TP53), genetic syndromes (e.g., Li-Fraumeni) or polymorphisms, and germline predispositions enhance susceptibility when combined with chronic inflammation.
- Environmental Triggers: The texture and particulate shedding from implants can modulate immune responses. Released silicone particles may further exacerbate local inflammation, amplifying the risk of lymphomagenesis.
Chronic inflammation resulting from textured implants highlights the importance of adopting smooth or innovative implant designs to minimize immune activation and reduce the risk of BIA-ALCL.
Risk Factors of BIA-ALCL
BIA-ALCL is rare, with a higher risk associated with macrotextured implants and cases typically emerging 8–10 years post-implantation.
Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) is linked to textured breast implants. Over 1,300 cases have been reported across 35 countries. The risk varies based on implant type, with Allergan Biocell implants carrying an estimated risk of 1:2,207, significantly higher than other textured implants (Clemens, 2019).
While the overall incidence remains low, specific implant types and patient factors significantly elevate risk. These include,
- Textured Implant Type: These carry a significantly higher risk compared to smooth implants. Increased surface area promotes bacterial colonization and biofilm formation, which may trigger chronic inflammation leading to lymphoma.
- History of Capsular Contracture: Persistent immune responses in the capsule region might act as a catalyst for malignant transformations.
- Genetic Predisposition: Mutations in pathways regulating immune responses have been associated with lymphoma development. Links to immune dysregulation syndromes like Li-Fraumeni syndrome are under investigation.
- Time Since Implantation: Most cases emerge 8–10 years post-implantation.
- Delayed Onset: Highlights the importance of long-term surveillance for patients with textured implants.
The long latency period underscores the importance of extended follow-up for patients, even in the absence of symptoms, to monitor for potential developments of BIA-ALCL.
Diagnosis of BIA-ALCL
BIA-ALCL diagnosis requires clinical evaluation, imaging, and confirmation via CD30 immunohistochemistry and flow cytometry.
BIA-ALCL is characterised by monoclonal CD30+ T-cells within a periprosthetic effusion or mass. This diagnosis is achieved with a triple assessment (clinical examination, imaging, and pathology).
Clinical Evaluation
BIA-ALCL typically presents 8–10 years after textured implant placement, with symptoms often arising from the luminal aspect of the peri-implant capsule.
Classic Findings
- Persistent seroma >1-year post-implantation, often rapid onset.
- Breast mass (~10–40% of cases) (Clemens, 2016).
- Breast asymmetry, pain, or palpable masses.
Delayed seromas occur in ~0.1 to 0.2 per- cent of patients following implantation of textured implants (McGuire, 2017).
Less Common Symptoms
- Lymphadenopathy, erythema, or skin lesions/ulceration (Adrada, 2014).
- Systemic “B” symptoms, such as unexplained weight loss, fever, or night sweats (~9% of cases) (Clemens, 2017).
A right-sided capsular contracture is illustrated below.

Rarely bilateral or incidental findings on routine histology post-capsulectomy (Miranda et al., 2014).
Imaging
Imaging confirms effusions, masses, and disease spread, essential for diagnosis and surgical planning.
- Ultrasound: First-line modality with sensitivity >80% for effusion detection; useful for assessing masses and lymphadenopathy (Adrada, 2014).
- MRI and PET/CT: Excellent for detecting implant integrity, capsular enhancement, and masses; aids in surgical planning
- Mammography: Limited utility due to low sensitivity and specificity; used to rule out primary breast cancer or other mimics
- FDG PET/CT: Recommended for staging and assessing systemic involvement. PET/CT aids in differentiating post-surgical inflammation from residual disease (Turton, 2020).
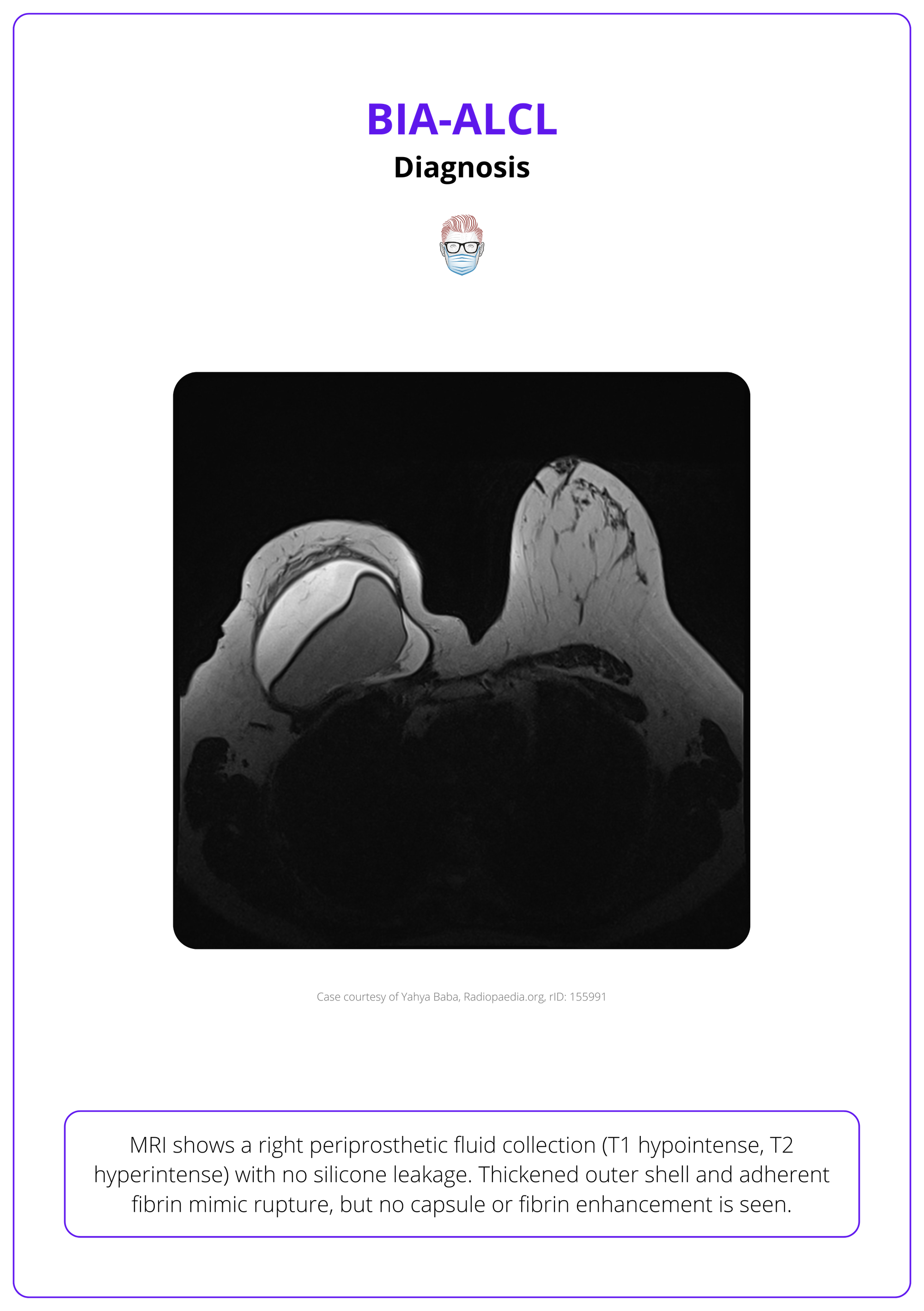
Pathological Confirmation
Pathological evaluation is critical for definitive diagnosis. Fluid aspirates and tissue biopsies undergo cytological, immunohistochemical, and molecular analysis.
- Cytology: Fine-needle aspiration (FNA) to collect peri-implant fluid, preferably ≥50 mL, to enhance diagnostic yield.
- Immunohistochemistry: Identifies CD30+ T-cells; absence of ALK translocations confirms BIA-ALC. Additional markers include CD2, CD3, CD4, TIA-1, and Granzyme B to confirm phenotype.
- Flow Cytometry: Detects T-cell clonality using T-cell receptor (TCR) gene rearrangement studies.
Differentials to rule out include silicone reactions, implant rupture, or benign late seroma or primary breast malignancies.
.
Staging of BIA-ALCL
The TNM staging system for solid tumors is preferred over traditional lymphoma staging methods for BIA-ALCL
Staging is critical for tailoring treatment strategies and predicting outcomes in BIA-ALCL. Unlike other lymphoid neoplasms, BIA-ALCL often presents as a localized disease confined to the implant capsule.
TNM Staging
The American Joint Committee on Cancer (AJCC) Tumor-Node-Metastasis (TNM) system provides a robust framework for classifying BIA-ALCL. It better reflects the localized and infiltrative nature of BIA-ALCL compared to the Lugano-modified Ann-Arbor classification.
Tumor (T)
- T1: Disease limited to effusion or the luminal side of the capsule.
- T2: Early infiltration into the capsule.
- T3: Dense aggregates or sheets of lymphoma cells breaching the capsule.
- T4: Lymphoma cells infiltrating beyond the capsule into surrounding soft tissue or breast parenchyma.
Node (N)
- N0: No lymph node involvement.
- N1: Single regional lymph node involvement.
- N2: Multiple regional lymph nodes are involved.
Metastasis (M)
- M0: No distant metastasis.
- M1: Distant organ involvement.
This can be visualised in the table below.

Key takeaway points from this classification are,
- Localized Disease (T1 or T2) is confined to the capsule or effusion. It has an excellent prognosis with complete surgical excision; up to 93% achieve complete remission within 2 years (Miranda, 2014).
- Aggressive Disease (T3, T4, N1, N2, or M1) extends beyond the capsule, involves lymph nodes, or metastasizes. Lower survival rates, requiring multimodal treatment (surgery, chemotherapy, radiotherapy).
Management of BIA-ALCL
Complete en bloc resection is the gold standard for preventing recurrence and achieving optimal outcomes. Advanced cases may require chemotherapy, radiotherapy, or both.
Management of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) emphasises complete surgical excision for localised disease, with systemic therapies reserved for advanced cases.
Surgical Management
The primary treatment for BIA-ALCL is surgical excision, which dramatically improves patient prognosis.
Total Capsulectomy
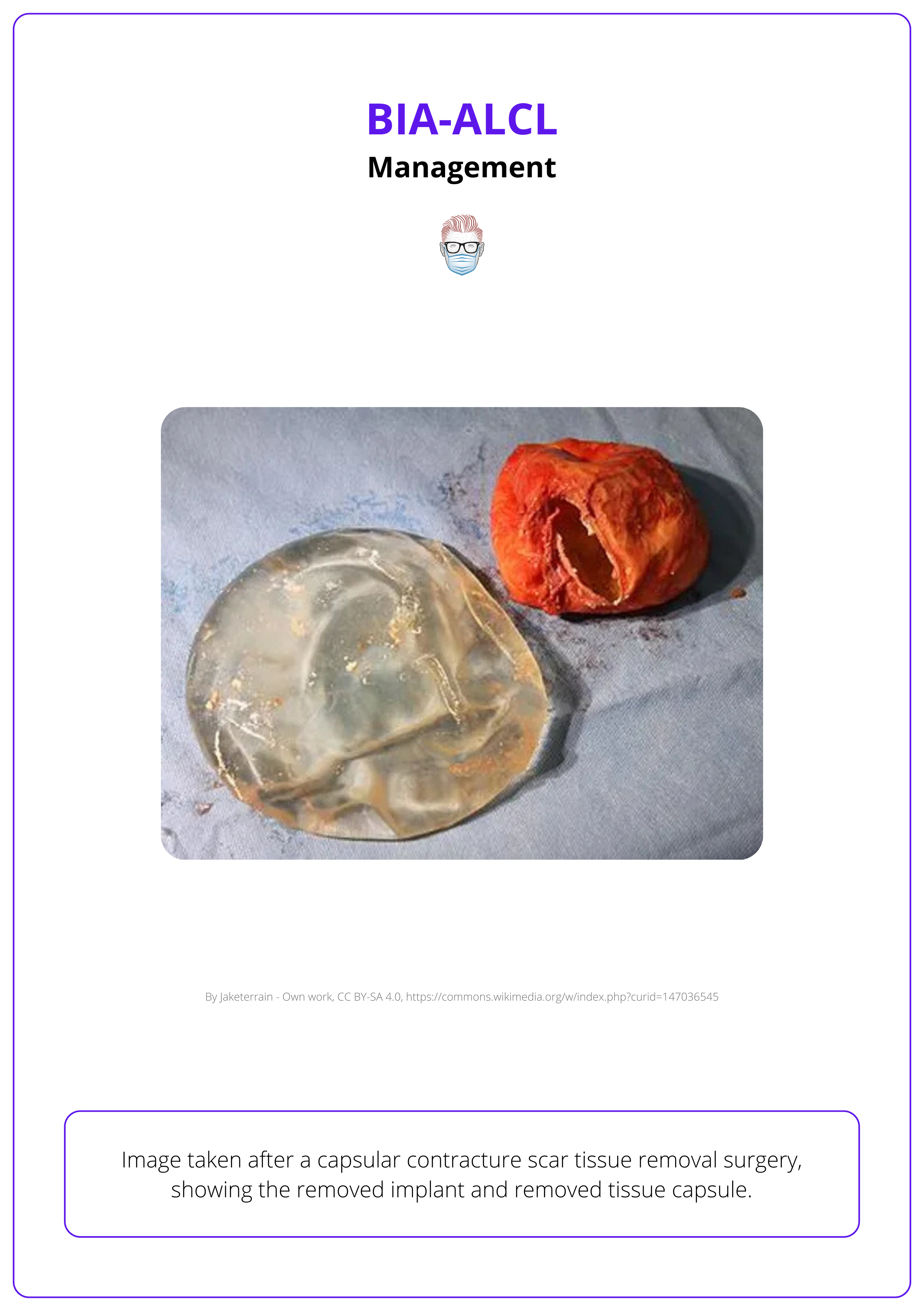
The primary surgical approach for managing BIA-ALCL is a total capsulectomy.
- Goal: Complete removal of the breast implant, the surrounding capsule, and any associated capsular mass.
- Technical Considerations: For retropectoral or dual-plane implants, adhesions to the ribcage may complicate resection. Tumescence of the anatomical plane can facilitate a safer and more effective capsulectomy.
Lymph Node Biopsy
- Suspicious Nodes: Excisional biopsy of involved or suspicious lymph nodes is essential to assess disease spread and guide treatment.
- Clinical Importance: Reduces recurrence risk by ensuring complete removal of potential sites of metastasis.
Prognostic Impact
- High Remission Rates: Complete en bloc resection with negative margins is strongly associated with favorable outcomes, including high remission rates for localized disease.
- Residual Disease Risks: Retained disease necessitates adjuvant chemotherapy, which can increase the treatment burden and associated risks.
While inadvertent spillage of seroma fluid during surgery is not clinically observed to influence recurrence rates, every effort should be made to minimize contamination.
Reconstruction Options
- Smooth Implants: Used to replace textured implants to mitigate recurrence risk.
- Autologous Tissue Reconstruction: Preferred for high-risk patients due to lower immunological reactivity.
- Timing: Reconstruction may be immediate or delayed depending on disease extent and patient condition.
Consider contralateral implant removal in high-risk cases, as incidental findings have been reported in 4.6% of cases (Clemens, 2017).
Advanced Cases and Adjuvant Therapy
Adjuvant therapies are essential for managing systemic disease or lymph node involvement (Ann Arbor Stage II or higher).
Chemotherapy
- Brentuximab vedotin, a CD30-directed monoclonal antibody, has shown effectiveness in refractory cases.
- Anthracycline-based regimens (e.g., CHOP: cyclophosphamide, doxorubicin, vincristine, prednisone) are used for systemic disease (Pro, 2012).
Radiotherapy
- Reserved for residual local disease post-surgery.
- Typical doses range from 24 to 36 Gy, depending on residual disease extent.
Surveillance and Prevention
Surveillance
- Clinical Follow-ups: Biannual checkups for the first two years post-treatment.
- Imaging: PET/CT is utilized as needed to monitor for residual or recurrent disease.
Prevention
- Smooth-Surfaced Implants: Avoid textured devices linked to higher risks.
- Sterile Surgical Techniques: Employ methods like the "no-touch" approach to minimize bacterial contamination and biofilm formation.
- Patient Education: Inform patients about early symptoms such as delayed seroma, mass formation, and systemic signs like fever or night sweats.
Conclusion
1. Classification and Overview: Gained a comprehensive understanding of BIA-ALCL, its classification, and key clinical features, including its association with textured breast implants.
2. Pathogenesis: Recognized the multifactorial nature of BIA-ALCL pathogenesis, involving chronic inflammation, bacterial biofilms, and genetic susceptibility.
3. Diagnosis: Learned the critical diagnostic steps, including clinical evaluation, imaging modalities, and pathological confirmation through CD30 immunohistochemistry.
4. Surgical Management: Identified the importance of complete en bloc resection and reconstruction strategies to achieve optimal outcomes.
5. Advanced Cases: Explored the role of chemotherapy and radiotherapy in treating advanced cases of BIA-ALCL with systemic involvement.
6. Surveillance and Prevention: Understood the value of regular follow-ups, patient education, and preventive strategies to minimize recurrence and improve long-term outcomes.


